Traditional510k: Most companies introducing a new Class II device must submit aTraditional 510k Premarket Notification. This is a full submission with21 sections. FDA’s clearance of a Traditional 510k is 90 days
Special510k: This clearance is used when there is a design change or amodification to a device you have previously cleared that does not affect theintended use. FDA’s clearance of a Special 510k is 30 days.
Abbreviated510k: There are specific guidelines for this 510k clearance. There has to be guidance documents from FDA for the specific medical devicethat is to be cleared. A special control has been established by FDA forthis device and consensus standards. FDA’s clearance of an Abbreviated510k is 90 days
FDA 510k SubmissionReqiurement
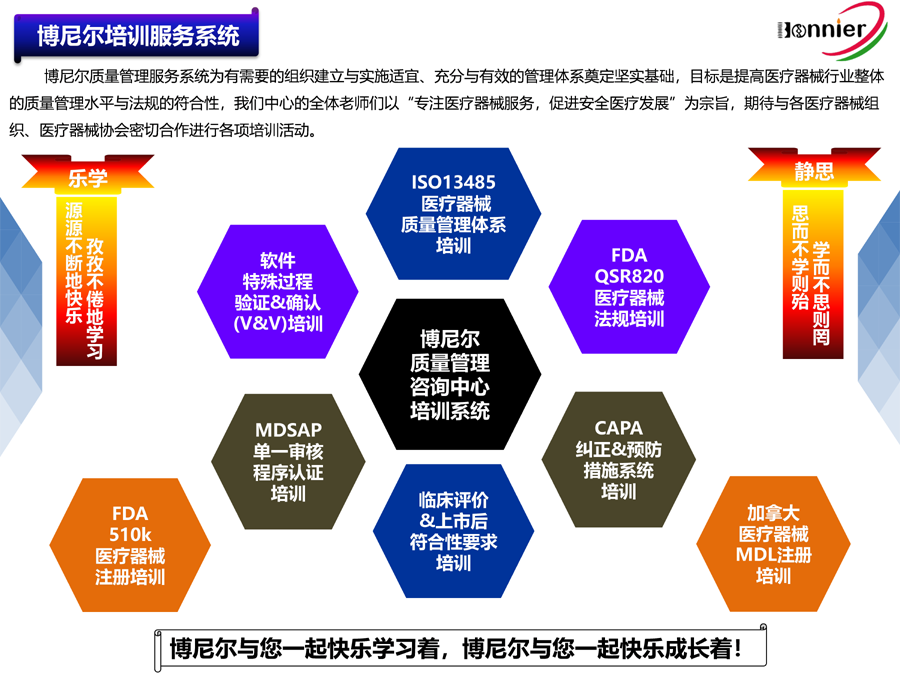
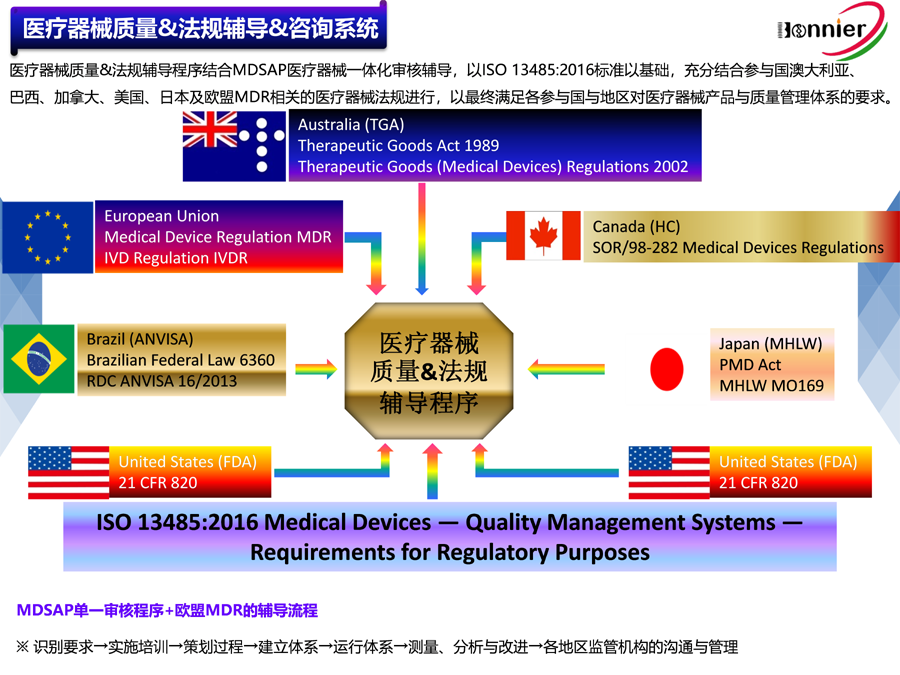
博尼尔质量管理咨询(江门)中心服务的内容:
※医疗器械注册咨询服务:
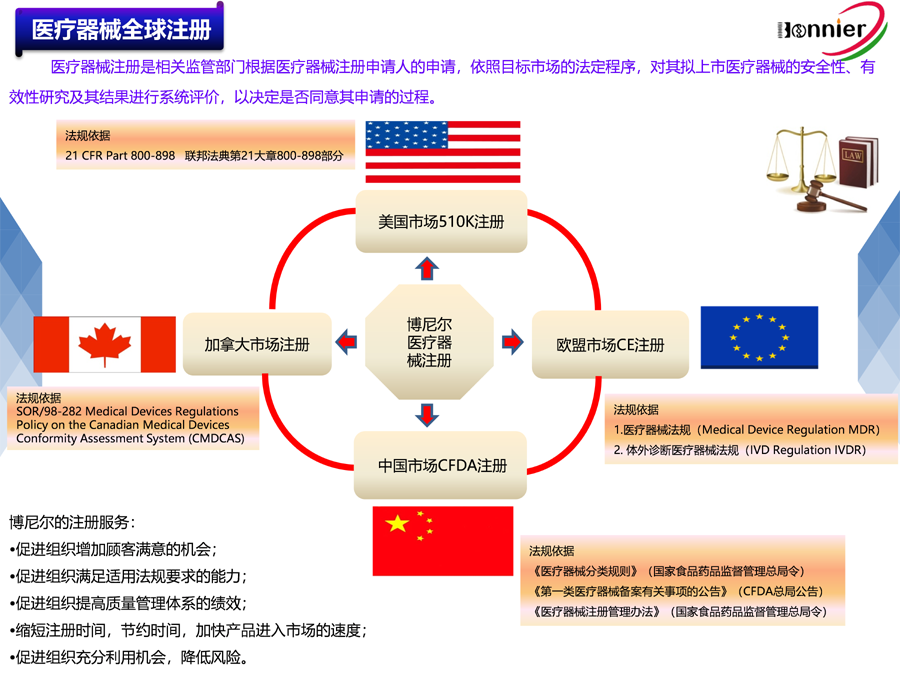
医疗器械FDA 510k咨询辅导;
医疗器械FDA 510k咨询;
医疗器械加拿大MDL注册咨询;
医疗器械MDR CE注册咨询。
※医疗器械质量管理体系咨询辅导服务:
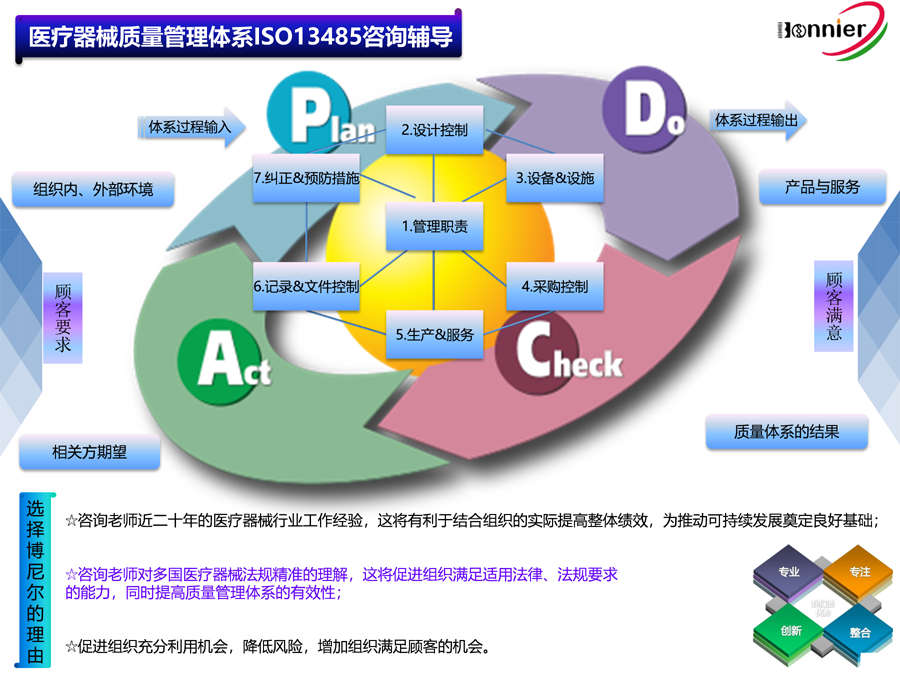
ISO13485咨询辅导;
医疗器械MDR CE咨询辅导;
医疗器械MDSAP认证咨询;
医疗器械QSR820咨询辅导。
※医疗器械授权代表服务:
英国授权代表UKRP;
瑞士授权代表CHREP;
美国代理人FDA Agent;
欧盟授权代表ECREP。